Publication of the team from the Laboratory of Experimental and Translational Immunology in the prestigious Journal of Extracellular Vesicles
We are pleased to inform that the article “Excess filaggrin in keratinocytes is removed by extracellular vesicles to prevent premature death and this mechanism can be hijacked by Staphylococcus aureus in a TLR2-dependent fashion” was published in the prestigious scientific journal Journal of Extracellular Vesicles, Impact factor equal to 17,337, 200 points of the Ministry of Education and Science.
The corresponding author of the publication is the head of the Laboratory of Experimental and Translational Immunology, dr hab. Danuta Gutowska-Owsiak, prof. UG, and the first author is a doctoral student, Mr. Adrian Kobiela. The co-authors are also current and former lab members, Dr. Lilit Hovhannisyan, Ms. Paulina Jurkowska, Mr. Argho Aninda Paul, Ms. Kinga Panek and Dr. Ewa Czechowska. The study also involved dr. hab. eng. Aleksandra Królicka, prof. UG (Laboratory of Biologically Active Compounds), dr. Michał Rychłowski (Department of Molecular Biology of Viruses) and dr. Aleksandra Bogucka (Laboratory of Mass Spectrometry). The work was carried out in cooperation with the University of Oxford and the Karolinska Institutet, Imperial College London and the Medical University of Gdańsk, and is available at the link:
https://onlinelibrary.wiley.com/doi/full/10.1002/jev2.12335
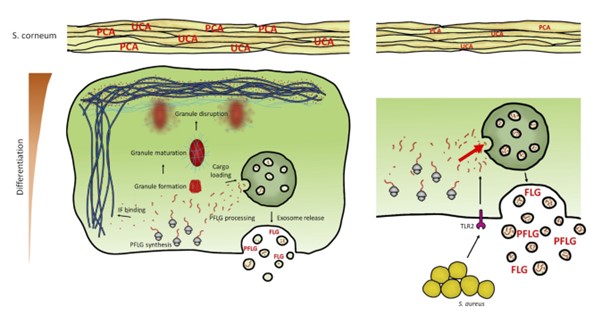
The skin of patients suffering from a chronic inflammatory skin disease, atopic dermatitis (AD), is characterized by a reduced level of filaggrin protein. Profilagrin (PFLG) is a protein produced mainly by highly differentiated keratinocytes and is necessary to maintain proper function of the epidermal barrier, e.g., strengthening it mechanically and providing antibacterial control through its breakdown products. In the upper layers of the epidermis, PFLG is proteolysed to active filaggrin monomers (FLG) that bind keratin fibers that make up the cytoskeleton of these cells. As a result of this process, keratinocytes die, forming stratum corneum, which supports impermeability of the upper layer of the skin and provides effective protection against harmful environmental factors.
With increased expression in the upper layers of the epidermis, PFLG forms keratohyalin granules (KHG), which enable spatial isolation of this protein in the cytoplasm, protecting cells from premature death. However, until now it was not known how this protein is expressed in the rest of the epidermis, and how the filaggrin content is controlled in its deeper, poorly differentiated layers, before the formation of KHGs. In our work, we showed that PFLG/FLG is already present in the layers of the epidermis containing poorly differentiated keratinocytes, but its amount is too small to form KHG, which could expose the cells to premature death. Our work has shown that the secretion of this protein by small secretory extracellular vesicles (sEVs) is a mechanism to control the level of PFLG/FLG in the cytoplasm, preventing from the premature cell death in the deepest layers of the epidermis, before the protein level allows the formation of granules.
Our research shows that peripheral blood sEVs from healthy donors as well as AD patients contain PFLG/FLG, which means that the protein can be transported from the skin to the bloodstream. Furthermore, we have also documented an increase in keratinocyte secretion of these FLG-containing vesicles by Staphylococcus aureus, a bacterium that often colonizes AD patients and affects their skin condition. In our work, we showed that these processes result from activation of the TLR2 receptor in keratinocytes, recognizing the bacteria. We believe that this mechanism may be hijacked by S. aureus as protection against the antimicrobial effects of FLG breakdown products released from keratinocytes damaged by infection.




